Stability testing of cosmetic products pdf
Stability of the cosmetic product (under reasonably foreseeable storage conditions) [Methods: Colipa (now Cosmetics Europe) and CTFA (now Personal Care Products Council)] Normally accelerated stability study is a 12 week test at 40°C and 75% relative humidity. Additional conditions are also recommended, if applicable: 4°C, 20°C daylight, 20°C dark, and 30°C. It could include a number of
medicinal products and illustrates these using two examples of different product types. Finally, a Finally, a proposal is made for a guideline on in-use stability testing, specifically for human
products should be labelled with ‘the expiry date of products with a stability of less than three years,’ but does not elaborate on this. The Cosmetic Products (Safety) …
In stability testing test attributes of BIWG 98 SE tablets 40 mg are investigated which are potentially susceptible to change during the course of storage, which are likely to …
Intertek personal care and cosmetic product stability testing helps ensure the product’s functionality and aesthetics are not adversely impacted during its intended shelf life and consumer use. Testing can be undertaken under controlled accelerated or real-time conditions.
The Complete Stability Testing for a Successful Application Strategies, Requirements, Basic Principles Performance, Documents 3. Performance . Performance 2 3.1. Stress and accelerated testing with the drug substance 1.2.1 Objective Elucidation of the intrinsic characteristics of the drug substance with reference to chemical properties (physical properties are investigated separately
DOWNLOAD GUIDELINES ON STABILITY TESTING OF COSMETIC PRODUCTS guidelines on stability testing pdf The testing should cover those features susceptible to change during storage and likely to influence quality,
regulated emulsion products must be tested in compliance with ap-plicable ICH guidelines. Further, although freeze-thaw has been widely used as an accelerated path to judge emulsion stability, it is not truly appropriate to rely solely on it to make those judgements. The cycling of temperature in freeze-thaw experiments affects parameters other than those that directly relate to creaming which
STABILITY TESTING OF COSMETIC PRODUCTS March 2004 I. GENERAL CONSIDERATIONS 1. INTRODUCTION General The purpose of stability testing cosmetic products is to ensure that a new or modified Fri, 01 Aug 2014 04:21:00 GMT GUIDELINES ON STABILITY TESTING OF COSMETIC PRODUCTS – Quality Control Best Practices Chapter 2 – Establishing and Managing an In-House …
Stability evaluation of organic Lip Balm SciELO

GUIDELINES FOR THE EVALUATION OF THE EFFICACY OF
Testing at 25°C/80% rh is not required if the product shows satisfactory stability during long term testing at 30°C/65% rh. If a product does not show satisfactory stability for at least 3 months at 40°C/75% rh, there are several acceptable options:
The claimed shelf life and storage conditions for each product should be derived from the results of the stability testing on that product. Generation of adequate stability data to support the assigned shelf life for a therapeutic sunscreen is the responsibility of the sponsor.
Cosmetic Products Regulation EC 1223/2009 requires a detailed safety assessment – the CPSR – before products can be marketed within the EU.
5.5.1 Testing parameters for different products shall be as per the Stability program. 5.5.2 The initial analysis data shall be taken from Certificate of analysis of the respective product and batch, provided the Date-in (To) of the stability study sample is within the …
cosmetic product. The stability of the cosmetics product under reasonably foreseeable storage conditions. 3. Microbiological quality The microbiological specifications of the substance or mixture and the cosmetic product. Particular attention shall be paid to cosmetics used around the eyes, on mucous membranes in general, on damaged skin, on children under three years of age, on elderly people
The purpose of stability testing is to ensure that the cosmetic product maintains its intended physical, chemical and microbiological quality, as well as functionality and aesthetics when stored under appropriate conditions.
Our cosmetic testing services further include a variety of protocols on preservation efficacy (challenge testing), patch testing, cosmetic efficacy, in vitro testing and stability testing. We also help you compile your product information file (PIF) and notify your products in the Cosmetic Products Notification Portal (CPNP).
9Characterize cosmetic products directly in original concentration at temperatures relevant for storage, transport and application. 9 Compare different formulations at different temperatures.
Cosmetics stability test can be performed in real time or it can be accelerated. The time that it takes to complete the test and the conditions under which it is performed can vary, as can the parameters that are monitored during the stability and compatibiltiy test.

The cosmetics industry has been micro testing products for many years and has a series of standard tests that you can use to get you started. Beyond that it may be worth talking to your micro laboratory (there are micro facilities all over the country as they have to test food, drink and medicines) about the specifics of your formulations – packaging, novel ingredients, stability etc. to see
Our stability testing for cosmetics is based on protocols designed to test the cosmetic product attributes that are susceptible to change during storage. These attributes may influence cosmetic product quality, safety and performance. We evaluate product quality at specific time intervals during storage under controlled conditions.
Many anhydrous products are mixtures of oils, esters, fatty alcohols, waxes, preservatives, fragrances and various pigments. Combining these materials is a complex undertaking, and varying the concentration even slightly changes the dynamics and aesthetics of the final product; in the worst cases, it causes instability.

As a result of stability testing a re-test period for the API (in exceptional cases, e.g. for unstable APIs, a shelf-life is given) or a shelf-life for the FPP can be established and …
Stability of Emulsions by Centrifugation and NMR we are able to obtain information for the long-term stability of emulsions in a relatively short period of time. PACS number: 82.70.Kj 1 Introduction One of the methods for evaluation of emulsion stability is to monitor the changes in the mean drop size for a certain period of storage time [1-3]. It is often con-sidered that an increase of
Safety Assessments for Cosmetics Safety and toxicological assessment consultancy and reporting. In the different cosmetics regulations worldwide a cosmetic safety assessment for human health is completed for cosmetic products.
Cosmetic products may include skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial make-up preparations, cleansing shampoos, permanent waves, hair colors, and deodorants, as well as any substance that is intended for use as a component of a
Cosmetics — Guidelines on the stability testing of cosmetic products ISO/TR 18811:2018 gives guidelines for the stability testing of cosmetic products. It reviews readily available bibliographic references that provide a resource for the assessment of the stability of cosmetic products.
Do cosmetic products also need to be stability tested with 3 batches from production line or can we prepare few samples ( 40 to 50 facial cream) in non-production line ( production line simulating system) system then use them for stability testing. Thanks
Stability of cosmetic formulations containing UV filters
It is the main ingredient of cosmetics formulation. Thus stability and quality of final product is Thus stability and quality of final product is dependent on the purity of water used so pure water should be used in manufacturing of
The rules governing cosmetic products in the European Union Volume 3 Guidelines Cosmetic products Notes of guidance for testing of cosmetic ingredients for their safety evaluation 1999 Edition EUROPEAN COMMISSION Enterprise Directorate-General Directorate-General Health and Consumer Protection. THE RULES GOVERNING COSMETIC PRODUCTS IN THE EUROPEAN UNION Volume 1 Cosmetics legislation Cosmetic
Cosmetics & Personal Care We can test and evaluate an unparalleled range of cosmetics, toiletries and raw materials for their efficacy, safety and regulatory compliance including those listed below, • Hair care: shampoos, conditioners and styling products such as … – a consumers dictionary of cosmetic ingredients adequate preservation, stability and compatibility issues, product scale-up and production problems, to cosmetic elegance considerations, such as fragrance selection, color, and packaging.
intended for use on children under the age of three and for cosmetic products intended exclusively for use in external intimate hygiene SCCNFP/0690/03, Final : Notes of Guidance for the testing of cosmetic
Stability Study of O/W Cosmetic Emulsions Using cosmetic products are meant only for the immediate surface of the skin . i.e. the epi- dermis. However, many commercial cosmetic products contain artificial chemicals that inevitably penetrate the skin. In the last few years physical products have started to gain the interest of consumers. Because of the incon- siderate use of chemical
Stability testing design A cosmetic stability test should be designed to assure Stability and physical integrity of cosmetic products under appropriate conditions of storage. The system we have described in our stability testing post meets these requirements. Back in 2004 the CTFA (now the PCPC) and its EU sister organization COLIPA issued some Guidelines on Stability Testing. Chemical
For cleansing products you’ll have to learn how to test foam. Moisturizing products will require you to learn how to judge skin condition after treatment. For makeup you’ll have to test formula “hardness” and ease of application. Ingredient Specialist You’ll also quickly learn which ingredients are essential to a formula and which are just “fluff” that support the marketing story
Light Stability Testing of Home and Personal Care Products Jeffrey Quill Director of Technical Applications . Q-Lab Corporation . Cleveland,Ohio . Headquarters & Instrument Division . Bolton, England Q-Lab Europe . Q-Lab Corporation • Founded in 1956 • Specialize in material durability testing equipment and services Shanghai, China Q-Lab China Saarbrücken Germany . Q-Lab was founded in
toiletry and perfumery industry cosmetics europe – the personal care association avenue herrmann-debroux 40.eu guidelines on stability testing of cosmetic products . +32 2 227 66 27 www. 1160 brussels t.cosmetics europe is the european trade association representing the interest of the cosmetics. +32 2 227 66 10.
Cosmetic stability predictions one of the key parameter for good quality of the product and is essential to assure the efficiency of the preservative system, and consumer safety. This study proposes the determination of cosmetics chemical stability validated by
the development of natural cosmetics and, within this category, organics (95% organic raw materials). The The image of environmentally friendly production is one of the strongest attractions of organic products.
Physical and physicochemical stability evaluation of cosmetic formulations 517 ration that presented the best performance in the previously mentioned screening study.
A cosmetic stability test should be designed to assure Stability and physical integrity of cosmetic products under appropriate conditions of storage, transport and use, Chemical stability,
Stress testing of the drug substance can help identify the likely degradation products, which can in turn help establish the degradation pathways and the intrinsic stability of the molecule and validate the stability indicating power of the analytical procedures used.
The purpose of stability testing cosmetic products is to ensure that a new or modified product meets the intended physical, chemical and microbiological quality standards as well as functionality and aesthetics when stored under appropriate conditions.
Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products Additional copies are available from: Office of Training and Communication
cosmetic product, it will still be necessary to conduct some toxicological testing with the complete formulation to assure adequately the safety of the finished cosmetic.”
guidelines on stability testing of cosmetic products Wed, 19 Dec 2018 18:31:00 GMT guidelines on stability testing of pdf – 1 GUIDELINES ON STABILITY TESTING OF
Shelf life of natural cosmeceutical product Accelerated
FDA Releases Q amp A on ANDAs and Stability Testing December 9th, 2018 – FDA has released answers to questions received during the public comment period for the draft guidance document Stability
The new EU Regulation 1223/2009 on Cosmetic Products Everything you need to know! SCC Ontario – March 25th, 2014 Marie Roussel & Ariane Divetain
guidelines on stability testing of cosmetic products Tue, 18 Dec 2018 16:44:00 GMT guidelines on stability testing of pdf – Stability Testing of New Drug
DOWNLOAD GUIDELINES ON STABILITY TESTING OF COSMETIC PRODUCTS guidelines on stability testing pdf 1 GUIDELINES ON STABILITY TESTING OF COSMETIC PRODUCTS March 2004 I. GENERAL
Stability Testing of New Drug Substances and Products 2 Stress testing of the drug substance can help identify the likely degradation products, which can in turn help establish the degradation pathways and the intrinsic stability of the molecule and validate the stability indicating power of the analytical procedures used. The nature of the stress testing will depend on the individual drug
ctfa guidelines on stability testing of cosmetic products Sun, 09 Dec 2018 20:01:00 GMT ctfa guidelines on stability testing pdf – 1 GUIDELINES ON
Evaluation of the Efficacy of Cosmetic Products. These guidelines aim to assist the cosmetics industry to comply with the applicable European regulations for the efficacy evaluation of cosmetic products.
The Complete Stability Testing for a Successful Application
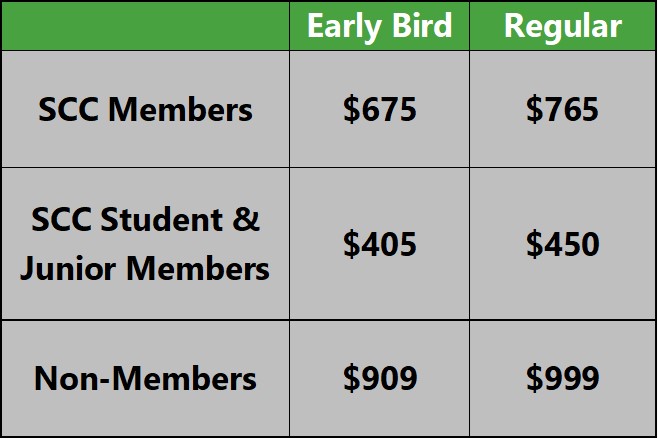
7. Stability testing Therapeutic Goods Administration (TGA)
Environmental Guidelines No. 10 2000 Vejledning fra Miljøstyrelsen Guideline on Safety assessment of cosmetic products Product information/dossier on cosmetic products,
This document, the “Notes of Guidance for Testing of Cosmetic Ingredients for Their Safety Evaluation ” represents a contribution of the members of the Scientific Committee on Cosmetic and Non-Food Products intended for consumers (SCCNFP), the former SCC
3.4 Testing of SPF and Labelling claims 43 3.5 Water Resistant testing and labelling claims 44 3.6 Stability Testing of Sunscreen Products 44 . 5 3.7 Consumer Information and advertising claims 45 3.8 Safety of the ingredients for use in sunscreen products 47 3.9 Animal testing 47 3.10 Reporting of Adverse Events 47 3.11 Good manufacturing practice 48 3.12 Nanoparticles 48 4 SUMMARY 48 . 6
Notes of guidance for testing os cosmetic ingredients for

Evaluation of Short-Term and Long-Term Stability of
Shelf – life of natural cosmeceutical product: Accelerated storaggye and stability test. Nur Zatul Iradah Bt. Roslan UTM INTERNATIONAL CAMPUSUTM INTERNATIONAL CAMPUS 8 OCTOBER 2010. Introduction Natural based formulation • Natural cosmetics have become a major trend in recent years • Theawarenessof naturalresourcesbasedproductis increasing. • Nt lNatural cosmetitics serve …
guidelines on stability testing of cosmetic products Fri, 09 Nov 2018 10:21:00 GMT guidelines on stability testing of pdf – Stability Testing of New Drug
Shelf life is commonly estimated using two types of stability testing: real-time stability tests and accelerated stability tests. In real-time stability testing, a product is stored at recommended storage conditions and monitored until it fails the specification. In accelerated stability tests, a product is stored at elevated stress conditions (such as temperature, humidity, and pH
In order to enhance consumer information PaO (Period after Opening) indication on the label aims to inform the consumer about the stability of the cosmetic, as it indicates the period of time after opening during which the product can be used without harmful effects.
guidelines on stability testing of cosmetic products Fri, 14 Dec 2018 12:39:00 GMT guidelines on stability testing of pdf – 1 GUIDELINES ON STABILITY TESTING OF
1 GUIDELINES ON STABILITY TESTING OF COSMETIC PRODUCTS March 2004 I. GENERAL CONSIDERATIONS 1. INTRODUCTION General The purpose of stability testing cosmetic products is to ensure that a new or modified
$ 50.00 – Guideline for Industry: The Stability Testing of Cosmetic Products (PDF Download) International Color Handbook, 4th Edition The Fourth Edition of the International Color Handbook assists international, regulatory and technical personnel in choosing a color palette to create the broadest range of cosmetic products.
of cosmetic products has become very extensive and is common in most population groups within the European Union, although the degree and nature may vary within the different Member States.
ICH Harmonised Tripartite Guideline The ICH Harmonized Tripartite Guideline covering the Stability Testing of New Drug Substances and Products (hereafter referred to as the Parent Guideline) notes that light testing should be an integral part of stress testing. This document is an annex to the Parent Guideline and addresses the recommendations for photostability testing. A. Preamble The
Stability Testing of Cosmetics Makingcosmetics.com
Online Store Personal Care Products Council
A Guide to United States Cosmetic Products Compliance
– SCCS’s Notes of guidance for the testing of cosmetic
COSMETIC REGULATION (EC) N° 1223/2009 sgs.com
guidelines on stability testing pdf valmaxindustries.com
Guidelines On Stability Testing Of Cosmetic Products




Intertek personal care and cosmetic product stability testing helps ensure the product’s functionality and aesthetics are not adversely impacted during its intended shelf life and consumer use. Testing can be undertaken under controlled accelerated or real-time conditions.
Guidelines On Stability Testing Of Cosmetic Products [PDF]
the development of natural cosmetics and, within this category, organics (95% organic raw materials). The The image of environmentally friendly production is one of the strongest attractions of organic products.
The new EU Regulation 1223/2009 on Cosmetic Products
Sunscreen Products Drug or Cosmetics
Preservative Efficacy Testing New Directions
Our cosmetic testing services further include a variety of protocols on preservation efficacy (challenge testing), patch testing, cosmetic efficacy, in vitro testing and stability testing. We also help you compile your product information file (PIF) and notify your products in the Cosmetic Products Notification Portal (CPNP).
Cosmetics- Guidelines on the stability testing of
guidelines on stability testing of cosmetic products Tue, 18 Dec 2018 16:44:00 GMT guidelines on stability testing of pdf – Stability Testing of New Drug
7. Stability testing Therapeutic Goods Administration (TGA)
The rules governing cosmetic products in the European Union Volume 3 Guidelines Cosmetic products Notes of guidance for testing of cosmetic ingredients for their safety evaluation 1999 Edition EUROPEAN COMMISSION Enterprise Directorate-General Directorate-General Health and Consumer Protection. THE RULES GOVERNING COSMETIC PRODUCTS IN THE EUROPEAN UNION Volume 1 Cosmetics legislation Cosmetic
Guidance for Industry Safety of Nanomaterials in Cosmetic
guidelines on stability testing pdf quizane.com
Stability testing design A cosmetic stability test should be designed to assure Stability and physical integrity of cosmetic products under appropriate conditions of storage. The system we have described in our stability testing post meets these requirements. Back in 2004 the CTFA (now the PCPC) and its EU sister organization COLIPA issued some Guidelines on Stability Testing. Chemical
guidelines on stability testing pdf quizane.com
Stability Testing for Personal Care and Cosmetic Products
A Guide to United States Cosmetic Products Compliance
This document, the “Notes of Guidance for Testing of Cosmetic Ingredients for Their Safety Evaluation ” represents a contribution of the members of the Scientific Committee on Cosmetic and Non-Food Products intended for consumers (SCCNFP), the former SCC
Cosmetics- Guidelines on the stability testing of
guidelines on stability testing pdf valmaxindustries.com
Guideline on Safety assessment of cosmetic products
Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products Additional copies are available from: Office of Training and Communication
A Guide to United States Cosmetic Products Compliance
The new EU Regulation 1223/2009 on Cosmetic Products
Guidelines On Stability Testing Of Cosmetic Products