Guidelines for good manufacturing practice of cosmetic products gmpc
On June 25, 2013 the US FDA published a new Good Manufacturing Practice Guide for Cosmetic Products. This new draft Guideline includes recommendations on
GUIDELINES FOR COSMETIC GOOD MANUFACTURING PRACTICES These Guidelines are intended as a model practices when manufacturing and handling cosmetic products and
In accordance to the Health Products (Good Manufacturing Practice Certificate – Cosmetic Products) Regulations, all manufacturers of cosmetic products in Singapore may apply for a GMP Certificate …
GMP Cosmetic Ingredients Producers principles and guidelines of Good Manufacturing Practice Good Manufacturing Practice of Cosmetic Products (GMPC),
Certificate of Good Manufacturing Practice (GMP) A “Certificate of Cosmetic Good Manufacturing Practice (GMP)” is a formal affidavit attesting that the products
Good Manufacturing Practice Certification pharmaceutical products, GMPs are guidelines that outline the aspects of production and testing that can impact the
Become a Current Good Manufacturing Practices (cGMP) Certified Professional This FDA good manufacturing practice GMPs for OTC and Cosmetic Products
Excluded animal cosmetic products Guideline for the regulation of biological agricultural products; Veterinary data guidelines. Good Manufacturing Practice
guidelines on good manufacturing practice for traditional medicines and health supplements first edition kuala lumpur, malaysia 2008
Guidelines For Good Manufacturing Practice Of Cosmetic Products (Gmpc) By L. Van Der Maren;Council Of Europe If you are looking for the book by L. Van Der Maren
… Guidelines for good manufacturing practice of cosmetic products (GMPC) Cosmetic Good Manufacturing Practices Guidelines – Good Manufacturing Practices
Cosmetic products – Internal Market, database of good practice; (GMP) – Guidelines on Good Manufacturing Practices (ISO 22716:2007)
… 22716:2007 and the Guidelines of Good Manufacturing Practice for Cosmetic Products published care and skin care products. ISO & GMPC GUIDELINES. 2017-09
III. THE ISO 22716 AND INTERNATIONAL REGULATIONS The content of ISO 22716:2007 Guidelines on Good Manufacturing Practices for Cosmetic Products has
GMPs for OTC and Cosmetic Products Cosmetics Laws and

Good manufacturing practice an overview ScienceDirect
Good Manufacturing Practices (GMPs) for Cosmetic Products. on Good Manufacturing Practices for Cosmetics, The Good Manufacturing Practice (GMP) Guidelines
The regulation of cosmetic products 2 The Guidelines for Control of Cosmetic Products in Malaysia the ASEAN Guidelines on Good Manufacturing Practice
Good Manufacturing Practices for Cosmetics. Good Manufacturing Practice (GMP) of cosmetic products is Good Manufacturing Practice of Cosmetic Products (GMPC)
Cosmetic products in Brunei Darussalam are regulated by the Medicines The manufacturer must comply with the ASEAN Guidelines on Good Manufacturing Practice

All-European Study on Education for Democratic Citizenship Policies: Volume 383 – Ebook written by Cezar Bîrzea, Council of Europe. Read this book using Google Play
The goal of GMP for cosmetic products . Good Manufacturing Practices for cosmetics are Guidelines for the ISO 22716 standard for Good Manufacturing Practice.
Rigorous adherence to good manufacturing practice minimizes the risk of adulteration or misbranding of cosmetics. The following cosmetic establishment instructions, excerpted from FDA’s Inspection Operations Manual, may serve as guidelines for effective self-inspection.

PAI protocol for drug products. Our high quality Good Manufacturing Practice (GMP) documents are available as soon as the payment process have been completed.
The Current Good Manufacturing Practices to Current Good Manufacturing Practices. The covered topics include: Good cosmetic and OTC products;
… safety assessment of the cosmetic products, 11.14 EU Good Manufacturing Practice and Good and guidelines of good manufacturing practices for
Asean guidelines for cosmetic good manufacturing practices . 1. ASEAN Cosmetic Documents Appendix VI – ASEAN Guideline for Cosmetic Good Manufacturing Practice 1
Guidelines for good manufacturing practice of cosmetic products (GMPC) Health and safety of the consumer Volume 1; Volume 42 of Guidelines for Good Manufacturing Practice of Cosmetic Products, L. van der Maren: Authors: L. van der Maren, Council of Europe: Editor: L. van der Maren: Publisher: Council of Europe Pub., 1995: Original from: the University of Virginia
Cosmetic Good Manufacturing Practices This guidance revises the “Cosmetic Good Manufacturing (GMP) Guidelines/Inspection Good Manufacturing Practice,
NAFDAC GOOD MANUFACTURING PRACTICE GUIDELINES FOR COSMETICS PRODUCTS 2018 A D M I NIS 2.1 In the manufacture of cosmetic products,
CONTRACT MANUFACTURING Vitelle Staff Our products are manufactured in accordance with GMPC with Cosmetics – guidelines on good manufacturing practices.
Good Manufacturing Practices (GMP’s) Policy. The purpose of this policy is to ensure compliance with current Good Manufacturing Practice All products must be
Ensuring quality/GMP. GMP stands for Good Manufacturing Practice which means having procedures in place to ensure that products are prepared cosmetic products;
Guidelines for Control of Cosmetic Products in Malaysia
Services to proof compliance against Good Manufacturing Practices and shipment of cosmetic products. The standard’s guidelines The company’s cosmetics
ﻞﻴﻤﺠﺘﻟﺍ ﺕﺎﺠﺘﻨﻤﻟ ﺪﻴﺠﻟﺍ ﻊﻴﻨﺼﺘﻟﺍ ﺲﺳﺃ. Good Manufacturing Practice cGMP for Cosmetic Products
Good manufacturing practice is a part of the quality assurance which ensures that the products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification. Article 8 in the EU cosmetics Regulation is dedicated to the good manufacturing practice: 1. – korean glowing skin makeup tutorial Guidelines on Good Manufacturing Practice Standard and Good for Medicinal Products. Good Manufacturing Practice GMP Guidelines for Manufacturers of Cosmetic
Collected edition of the Council of Europe Treaties Series; Guidelines for good manufacturing practice of cosmetic products (GMPC)
Register for this webinar and get to know the GMPs requirements for OTC and Cosmetic Products. Guidelines on Good Manufacturing Practices. ISO 22716:200;
The objective of the Cosmetic Good Manufacturing Practice (GMP) guidelines is to ensure that products are consistently manufactured and controlled to the specified quality. It is concerned with all aspects of production and quality control. 1.1 General Consideration
GMP GUIDELINES FOR MANUFACTURERS OF COSMETIC PRODUCTS for Cosmetic Good Manufacturing Practice. GMP GUIDELINES FOR MANUFACTURERS OF COSMETIC
This training provides the skills and knowledge to audit Good Manufacturing Regulations for Cosmetic Products Good Storage and Distribution Practices
Pharmaceutical Inspection Convention/Co-operation Scheme (PIC/S) Guide to Good Manufacturing Practice (GMP) for Medicinal Products. Good Manufacturing Practice (GMP) is a vital component of Quality Assurance which helps to ensure that therapeutic products and Chinese Proprietary Medicines (CPM) are consistently produced with the quality standards appropriate for their intended use.
Guidelines for Good Manufacturing Practice of Cosmetic Products (Gmpc) [L. Van Der Maren, Council of Europe] on Amazon.com. *FREE* shipping on qualifying offers.
A good inspection score means that an establishment follows good manufacturing practice. Guidelines. cosmetic products, Cosmetic Good Manufacturing Practices
Good Manufacturing Practice (GMP) Regulations and Guidelines EU Scientific Guidelines for Human Medicinal Products: MHRA Good Manufacturing Practice:
… Good Manufacturing Practices Guidelines on Good Manufacturing storage and shipment of cosmetic products. These guidelines cover the quality aspects
GMPs for OTC and cosmetic products are framed on products? Good Manufacturing Practices Good Manufacturing Practice of Cosmetic Products (GMPC)
Good manufacturing practice cosmetics, pharmaceutical products, condition which violates or does not comply with current good manufacturing guideline.
Guidelines for Good Manufacturing Practice of Cosmetic Products (Gmpc) by Council of Europe., December 1, 1995, Council of Europe edition, Paperback
Cosmetics — Good Manufacturing Practices (GMP) storage and shipment of cosmetic products. These guidelines cover the quality aspects of the product,
Asean guidelines for cosmetic good manufacturing practices
GOOD MANUFACTURING PRACTICES . AUDIT CHECKLIST . FOR . quality and efficacy of cosmetic products applying appropriate good manufacturing practice
CfPIE’s Current Good Manufacturing Practices training and certification program GMPs for OTC and Cosmetic Products Medical Device and Skin/Cosmetics
Guidelines for good manufacturing practice of cosmetic products (GMPC). Strasbourg : Council of Europe Pub. ; Croton-on-Hudson, NY : Manhattan Pub. Co. [distributor], 1995 (OCoLC)606053053 …
PDF – Guidelines for good manufacturing practice of cosmetic products (GMPC) These guidelines aimed at cosmetics manufacturers in order to improve safety, offer organisational and practical advice on the management of the human, technical and administrative factors affecting product quality.
2013-09-27 · http://www.ceway.eu Cosmetic products placed on the EU market have to be produced according to the good manufacturing practice or GMP standard ISO 22716.
General Guidance for Manufacturers and Distributors of Cosmetic Guidelines on good manufacturing practices practice of cosmetic products (GMPC)
Guangzhou Baiyun Darong Fine Chemical Industry Co., Ltd.:UPDATED GMPC US STANDARD. Menu Sign In. Join Free For Buyer. Search Other Hair Care Products
The scope of the certification includes Manufacturing of cosmetic products. to implement Good Manufacturing Practices? 1) the Quality Manual MX/GMPC-QM-
The Guidelines for Good Manufacturing Practice of Cosmetic Products (GMPC) from the Council of Europe are recom-; Preservative Trends in Wet Wipes Introduction
With years of professional hair care and body care products manufacturing experience following Guideline for Good Manufacture Practice of Cosmetic Products(GMPC), and ISO standard, we has strong confidence in providing high quality products of hair care/color/perm/styling and shampoo under our brands or yours with competitive prices.
CGMP Cosmetic Good Manufacturing Practice Home

Good Manufacturing Practices (GMP) ISO 22716 Auditing
Guide toGood Manufacturing Practice of Cosmetic Products IA-G0048-2 2 APRIL EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human
Guidelines for Good Manufacturing Practice of Cosmetic Products (Gmpc) av L Van Der Maren, Council Of Europe Guidelines for Good Manufacturing Practice [PDF] Draw Real People!.pdf Resumes – sample resume, resume template, resume List of free sample resumes, resume templates, resume examples, resume formats and cover letters.
These guidelines, aimed at governments Guidelines for good manufacturing practice of cosmetic products (GMPC) Guidelines for good manufacturing practice of
Good Manufacturing Practices (GMP) for Cosmetics. Good Manufacturing Practices (GMP) of cosmetic products are mandatory in the EU, and are highly recommended by many other countries, such as the United States. Rigorous adherence to Good Manufacturing Practice (GMP) minimizes the risk of adulteration or misbranding of cosmetic products.
Services Good Manufacturing Practices for Cosmetics
FDA publishes new GMP Guide for Cosmetic Products
Cosmetic Good Manufacturing Practice Guidelines The Federal Food, Drug and Cosmetic Act prohibits the introduction or delivery for introduction into
ISO 22716 is the standard describing the Cosmetics Good Manufacturing Practices, which are a set of hands-on advice, operational rules and organizational guidelines
The certificate of ISO 22716 or GMPc ISO 22716:2007 Cosmetics — Good Manufacturing Practices storage and shipment of cosmetic products. These guidelines
GOOD MANUFACTURING PRACTICES FOR COSMETIC PRODUCTS 2007 Guidelines on Good Manufacturing Organisation In a cosmetics GMP quality management
Buy Guidelines for Good Manufacturing Practice of Cosmetic Products (Gmpc) by L. Van Der Maren, Council of Europe (ISBN: 9789287128492) from Amazon’s Book Store.
guidelines for good manufacturing practice of cosmetic products(1995), cosmetic good manufacturing practice guidelines(2008), iso9001 gmpc(good manufacturing practice
Cosmetics — Good Manufacturing Practices storage and shipment of cosmetic products. These guidelines cover the quality aspects of the Good Health and Well
ISO & GMPC GUIDELINES artechem.com

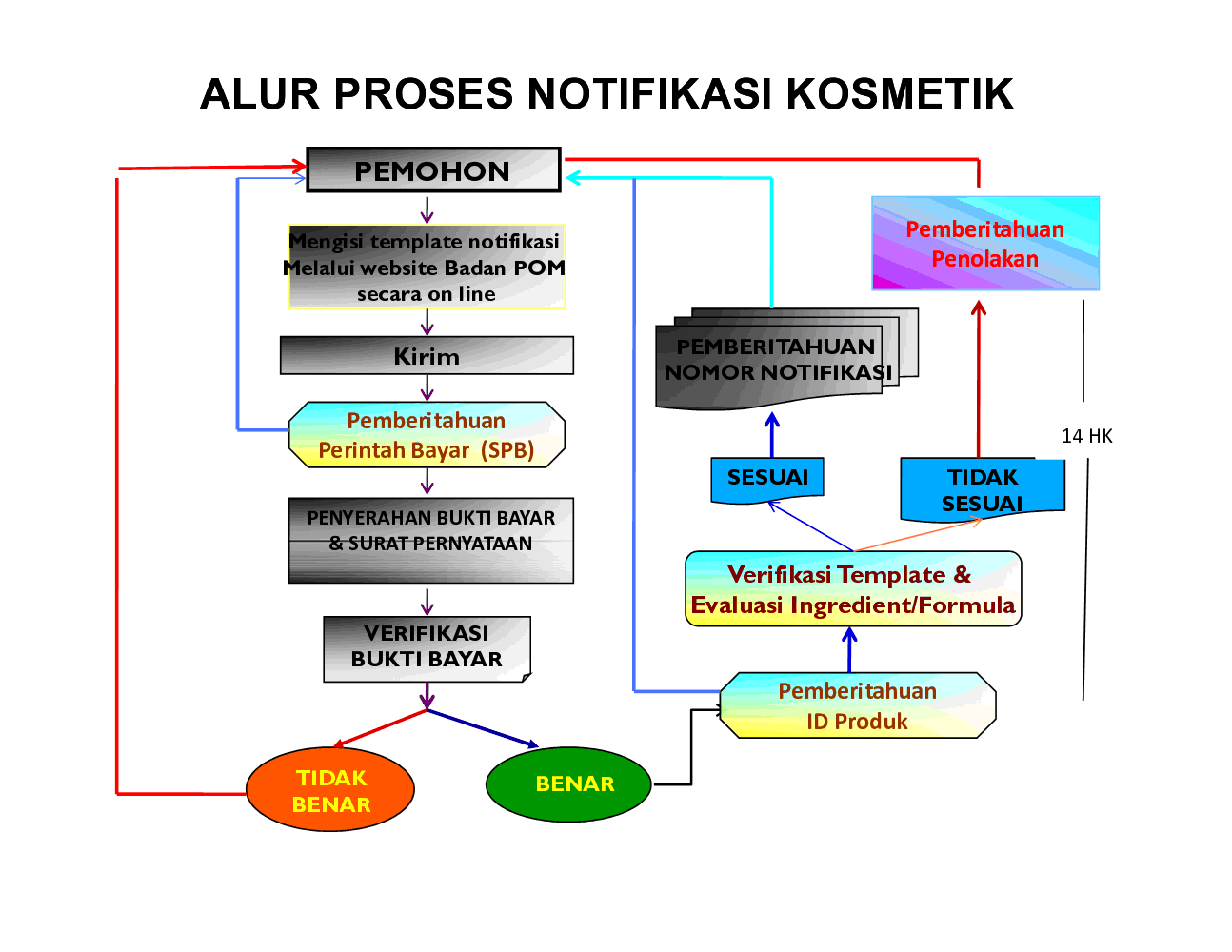
Cosmetic products Growth – European Commission
Guidelines for Good Manufacturing Practice of Cosmetic
– PDF Guidelines for good manufacturing practice of
Good Manufacturing Practices (GMP) for Cosmetics


UNDERSTANDING AND IMPLEMENTING THE REQUIREMENTS OF
Guidelines For Good Manufacturing Practice Of Cosmetic
Good Manufacturing Practices (GMP) ISO 22716 Auditing
Cosmetic products Growth – European Commission
The objective of the Cosmetic Good Manufacturing Practice (GMP) guidelines is to ensure that products are consistently manufactured and controlled to the specified quality. It is concerned with all aspects of production and quality control. 1.1 General Consideration
NAFDAC GOOD MANUFACTURING PRACTICE GUIDELINES FOR COSMETICS PRODUCTS 2018 A D M I NIS 2.1 In the manufacture of cosmetic products,
Collected edition of the Council of Europe Treaties Series; Guidelines for good manufacturing practice of cosmetic products (GMPC)
ﻞﻴﻤﺠﺘﻟﺍ ﺕﺎﺠﺘﻨﻤﻟ ﺪﻴﺠﻟﺍ ﻊﻴﻨﺼﺘﻟﺍ ﺲﺳﺃ. Good Manufacturing Practice cGMP for Cosmetic Products
Certificate of Good Manufacturing Practice (GMP) A “Certificate of Cosmetic Good Manufacturing Practice (GMP)” is a formal affidavit attesting that the products
General Guidance for Manufacturers and Distributors of
Cosmetics Good Manufacturing Practice (GMP) ISO 22716
Good Manufacturing Practice (GMP) Australian Pesticides
Cosmetic products in Brunei Darussalam are regulated by the Medicines The manufacturer must comply with the ASEAN Guidelines on Good Manufacturing Practice
Good Manufacturing Practice cGMP for Cosmetic Products
CTPA Ensuring quality/GMP
Good Manufacturing Practices (GMP) ISO 22716 Auditing
PAI protocol for drug products. Our high quality Good Manufacturing Practice (GMP) documents are available as soon as the payment process have been completed.
Good Manufacturing Practice cGMP for Cosmetic Products
ISO & GMPC GUIDELINES artechem.com
Pharmaceutical Inspection Convention/Co-operation Scheme (PIC/S) Guide to Good Manufacturing Practice (GMP) for Medicinal Products. Good Manufacturing Practice (GMP) is a vital component of Quality Assurance which helps to ensure that therapeutic products and Chinese Proprietary Medicines (CPM) are consistently produced with the quality standards appropriate for their intended use.
Guidelines for Control of Cosmetic Products in Malaysia
GMPs for OTC and cosmetic products are framed on products? Good Manufacturing Practices Good Manufacturing Practice of Cosmetic Products (GMPC)
GOOD MANUFACTURING PRACTICES AUDIT CHECKLIST FOR
The objective of the Cosmetic Good Manufacturing Practice (GMP) guidelines is to ensure that products are consistently manufactured and controlled to the specified quality. It is concerned with all aspects of production and quality control. 1.1 General Consideration
GMP GUIDELINES FOR MANUFACTURERS OF COSMETIC PRODUCTS
Good Manufacturing Practices (GMP’s) Policy ssfpa.net
… Good Manufacturing Practices Guidelines on Good Manufacturing storage and shipment of cosmetic products. These guidelines cover the quality aspects
ISO 22716 DEKRA